Cord blood herapy is gaining significant attention this Cord Blood Awareness Month as Australia records its first use of cord blood to treat a child with cerebral palsy outside a clinical trial.
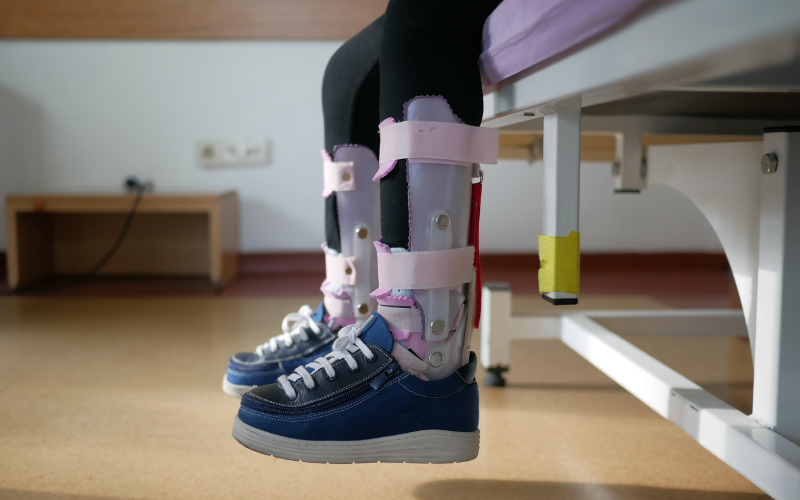
In April 2025, Zara became the first child in Australia to receive cord blood therapy for cerebral palsy outside of a clinical trial. Under the supervision of Dr. Michael Fahey, a pediatric neurologist at Monash Children’s Hospital, Zara was infused with her own umbilical cord blood — a pioneering treatment in the country.
This groundbreaking procedure was made possible through the Therapeutic Goods Administration (TGA)’s Special Access Scheme (SAS) — Category B. This compassionate access pathway allows physicians to apply on behalf of their patients to use therapies that are not yet widely approved. Upon approval, children with cerebral palsy can receive cord blood therapy locally, with treatment costs typically covered by the national healthcare system.
The Cerebral Palsy Alliance (CPA) of Australia played a crucial role in enabling this advancement. CPA, known for its research and advocacy in the cerebral palsy community, has been instrumental in sharing scientific evidence on cord blood therapy’s safety and efficacy. In April 2025, CPA led a multinational collaboration that published a study in Pediatrics, demonstrating that cord blood therapy can notably improve motor skills in some children with cerebral palsy.
Megan Finch-Edmondson, PhD, the lead author of the study, highlights the benefits of cord blood therapy: “Cord blood is fantastic because it is backed now by strong scientific evidence to show that it not only is safe, but it also can provide benefit for children living with cerebral palsy.” She explains that the cells in cord blood release factors that help repair brain injury — the underlying cause of cerebral palsy.
To support families and healthcare providers interested in this treatment, CPA has set up a dedicated webpage to guide access to cord blood therapy. CPA continues to publish research and assist families in connecting with clinicians who support compassionate use access.
Currently, Zara’s treatment is the only approved compassionate use case in Australia. Access to cord blood therapy under this scheme is limited to Australian citizens due to national healthcare funding policies. Families must also have stored their own cord blood — either from the treated child or a compatible sibling — in compliance with regulations governing public cord blood banks. Although much research supports the use of donor cord blood from public banks, these regulations limit wider access in Australia.
This Cord Blood Awareness Month, Zara’s story reminds us of the importance of cord blood banking and the evolving role of cord blood therapy in treating cerebral palsy.
Want to learn more about cord blood banking?
Contact us at +97143116613 or Download a Free Info Pack
Reference: Australia Allows Umbilical Cord Blood Therapy for Cerebral Palsy | Parentsguidetocordblood.org
